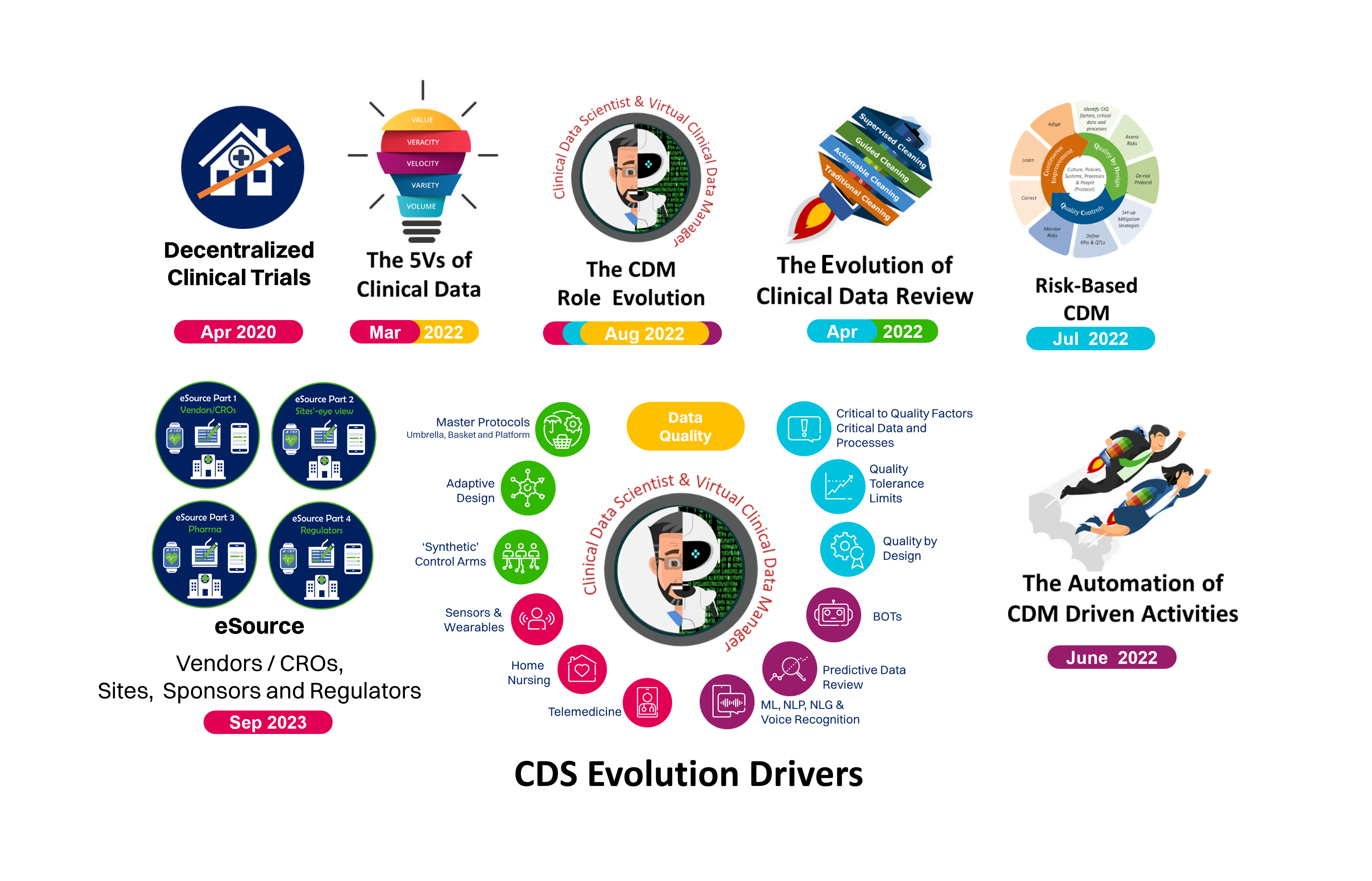
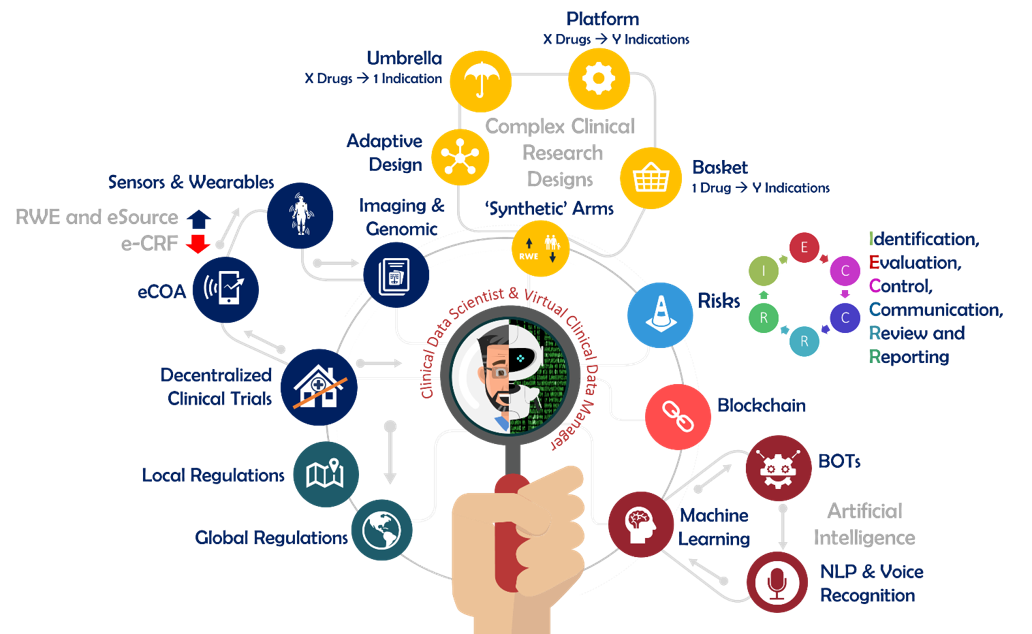
Nearly five years ago, SCDM underscored the need to reshape and rethink the CDM profession in light of key industry drivers and trends in Clinical Research. Ever since, we’ve been at the forefront of guiding the evolution from Clinical Data Management to Clinical Data Science through our thought leadership, providing leaders and organizations with insights and associated best practices for the CDM role transition journey.
THOUGHT LEADERSHIP & CDS
Leading the
industry-wide transition
to Clinical Data Science
Proudly advancing Clinical Data Science
FROM VISION TO REALITY
How to create a Clinical Data Science organization
After several reflection papers and topic briefs on the on the evolution of Clinical data Management (CDM) toward Clinical Data Science (CDS), we are transforming the vision into reality. This position paper clarifies key CDS concepts and provides insights on how CDM professionals can efficiently set their path toward CDS and make it happen!
While this paper is not meant to be an exhaustive change management guide, it provides a concrete set of recommendations on how to evolve an organization toward CDS or simply build/rebuild a CDM organization.
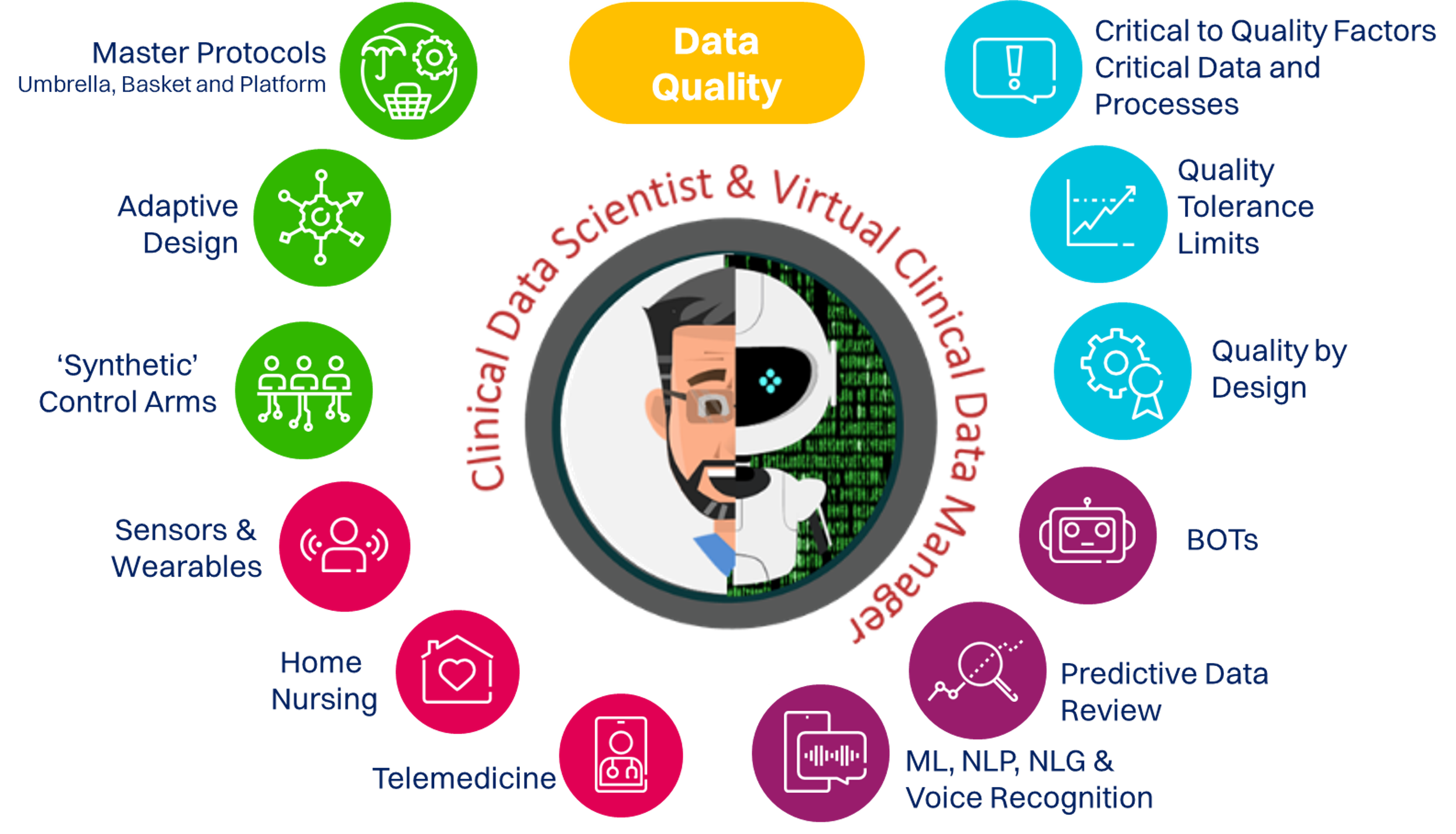
ON-DEMAND WEBINAR
Clinical Data Science 101
As CDM professionals embark on their journey towards Clinical Data Science (CDS), it is crucial to understand what CDS is and what it is NOT. Only then you will be able to assess its impact on you and your organization.

PUBLICATIONS
The Evolution of Clinical Data Management into Clinical Data Science
Definition of CDS
First, Clinical Data Science is not to be confused with the general discipline of Data Science which applies across multiple industries. From an SCDM point of view and as expressed in the third reflection paper, Clinical Data Science is an evolution of Clinical Data Management. Clinical Data Science encompasses processes, domain expertise, technologies, data analytics and Good Clinical Data Management Practices essential to prompt decision making throughout the life cycle of Clinical Research. Clinical Data Science can be defined as the strategic discipline enabling the execution of complex protocol designs in a patient centric, data driven and risk-based approach ensuring subject protection as well as the reliability and credibility of trial results.
In contrast, Clinical Data Management is responsible for the life cycle of clinical data from collection to their delivery for statistical analysis in support of regulatory activities. Clinical Data Management is primarily focusing on dataflows and data integrity (i.e., data is managed the right way). Clinical Data Science broadens this focus by adding the data risk, data meaning and value dimensions for achieving data quality (i.e., data is credible and reliable). Clinical Data Science also expands the scope of Clinical Data Management beyond the study construct by requiring the ability to generate knowledge and insights from clinical data to support other clinical research activities which requires different expertise, approaches, and technologies.
Part 1: Drivers
A Reflection Paper on the impact of the Clinical Research industry trends on Clinical Data Management
The main objective of this paper is to provide a forward‐looking and pragmatic view on why and how emerging study designs, regulations and technology innovations are reshaping the role and profile of CDM.
Part 2: The technology enablers
A Reflection Paper on how technology will enable the evolution of Clinical Data Management into Clinical Data Science
This second paper focuses on the technologies enabling our evolution towards Clinical Data Science and allowing us to efficiently manage the 5Vs of clinical data (i.e., Variety, Volume, Velocity, Veracity and Value). It shares insights and lessons learned from leaders, pioneers and early adopters of those emerging technologies.
Part 3: The evolution of the CDM role
A Reflection Paper on the evolution of CDM skillsets and competencies
This paper provide insights on how CDM professionals who have successfully and passionately contributed to the credibility of CDM can evolve their skillsets and competencies to cope with the increasing complexities of clinical research which demands novel approaches maximizing the potential of available technologies.
Applied Clinical Trials (March 2020) – The Clinical Data Manager: A Roadmap for the Future
Clinical Leader (September 2020), What Is Your AI Road Map To Revolutionize Drug Development?
An Industry Position Paper on the use of Audit Trail Review (ATR) as a key tool to ensure data integrity

Cat Hall
Vice President, Product Strategy, endpoint Clinical

Catherine Celingant
Executive Director, Data Monitoring and Management, Pfizer

Demetris Zambas
Vice President and Global Head of Data Monitoring and Management, Pfizer

François Torche
CEO, CluePoints

Ian Shafer
Partner, PwC

Inder Sachdeva
Portfolio Delivery Lead, Clinical Data Sciences & Safety, Cognizant Technology Solutions

Josh Wilson
Executive Director, Strategic Technology Advancement, Biometrics, Syneos Health

Lynne Cesario
Global Risk Based Monitoring Program Lead, Pfizer

Patrick Nadolny
Global Head, Clinical Data Management, Sanofi

Prasanna Rao
Sr. Director, Artificial Intelligence and Machine Learning, Pfizer

Richard Young
Advocating the need for data management every day

Sanjay Bhardwaj
Head of Clinical Technology Strategy & Operations, Abbvie

Steve Chartier
Sr. Director of Software Engineering, Calyx
Be the first to know
Sign up to get our latest news and industry updates.